Transcranial Magnetic Stimulation
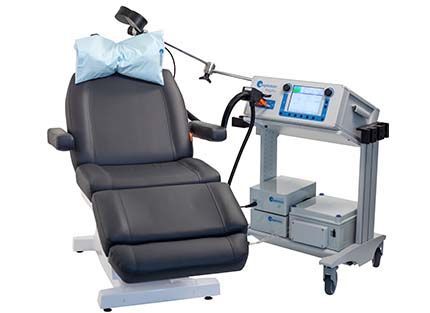
What is TMS?
Transcranial Magnetic Stimulation (TMS) was approved in 2008 by the FDA to be an effective therapy for treatment-resistant depression.
Depression affects over 18 million adults in the United States every year. People suffering from depression experience a significant loss in pleasure or interest in activities for a prolonged period of time. Severe depression lasts longer and has results in significant impairment in family, personal, social, occupational, educational, and/or other important areas of functioning.
TMS works by delivering electromagnetic pulses through a figure-8 coil placed specifically on the scalp to target the dorsolateral prefrontal cortex (DLPFC). This is the area of the brain that research has shown to be under active in the brains of patients with depression, and is involved in executive functioning, working memory, and spatial attention.
How it works
Contact us
Let us know you're interested in receiving TMS for Major Depression and we will begin the intake process, which will include consent forms, releases of information (ROIs) and scheduling an evaluation with our provider team.
Evaluation
Our provider team will sit down with you and go over your records with you as well as discuss with you the benefits you can hope to see with TMS.
Intitial mapping
Our trained technician will conduct the initial brain mapping so as to custom tailor your TMS treatment for you, as well as conduct the first treatment session while you sit comfortably in the treatment chair.
Treatment and follow-ups
Treatments take approximately 19 minutes, once per day, five days per week for the first six weeks, followed by a tapering period lasting three weeks. Your provider will check in with you regularly to see how you are responding to treatment.